FDA PCR Molecular (OTC) Self Test
The Food and Drug Administration (FDA) has issued an Emergency Use Authorization (EUA) for then Lucira Check It™ COVID-19 test kit (Lucira Health, Inc), an over the-counter (OTC) single-use molecular test to detect COVID-19 in individuals with or without symptoms.
The Lucira Check It COVID-19 test kit utilizes a molecular amplification technology to detect SARS-COV-2 RNA. The EUA allows for nasal swab samples to be self-collected at home by an individual 14 years of age and older or by an adult from an individual 2 to 13 years of age.
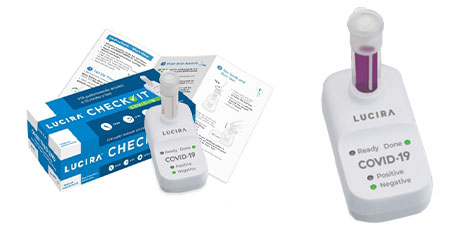
Each test kit includes the test device, sample vial, nasal swab, and 2 AA batteries to run 1 COVID-19 test. After the nasal swab is rotated in each nostril 5 times, the swab is stirred in the sample vial, which is then pressed into the test unit. The “ready” light will blink until a “positive” or “negative” green light is illuminated. Results are provided as quickly as 11 minutes for a positive result and 30 minutes for a negative result.
Findings from 2 Community Testing studies showed that the Lucira test was 98% accurate when compared with the FDA-authorized Hologic Panther Fusion PCR test; the comparative positive result agreement was 97% and the negative result agreement was 98%. Accuracy was observed to be 96% when 10 samples with very low viral loads were included in the data.

