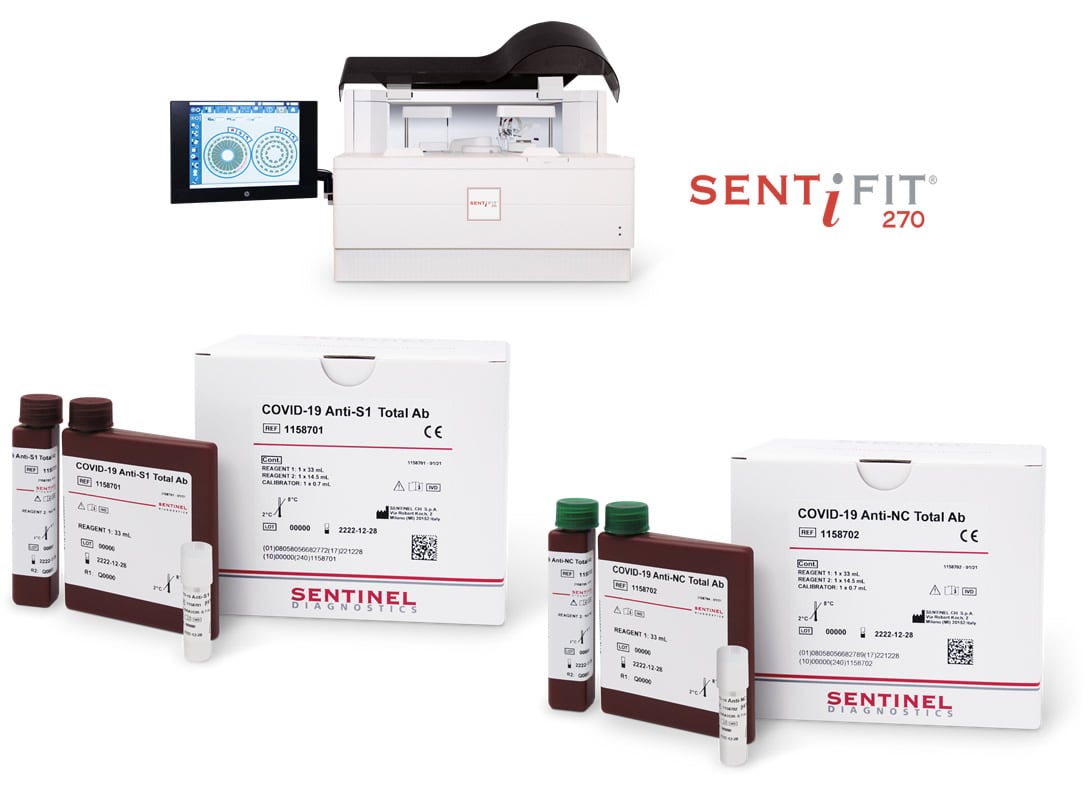
Sierologic Covid 19 Vaccine Test Kit : Anti-NC Total AB & Anti-S1 Total AB
The SENTINEL DIAGNOSTICS solution
The quick complete platform for quantitative detection of Total Covid-19 Antibodies
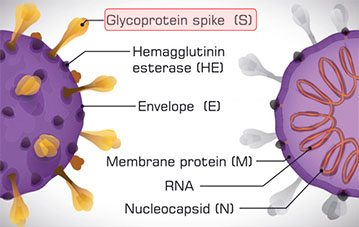
COVID-19 Anti-S1 Total Ab
Immunoturbidimetric test for the quantitative and qualitative detection of human total antibodies (IgA, IgG and IgM) specific for Spike RBD protein S1 of SARS CoV-2 in human serum
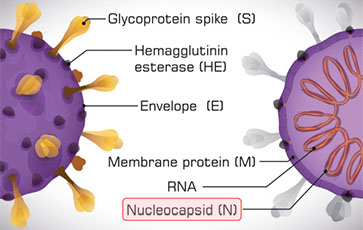
COVID-19 Anti-NC Total Ab
Immunoturbidimetric test for the quantitative and qualitative detection of human total antibodies (IgA, IgG and IgM) specific for NC protein of SARS-CoV-2 in human serum
Detection both S1 and NC Abs gives more info to better answer
| COVID-19 Anti-S1 Total Ab | COVID-19 Anti-NC Total Ab | Type of SARS-CoV-2 antibodies | Results interpretation |
|---|---|---|---|
| + | + | Presence of – Neutralizing antibodies – Non-neutralizing antibodies |
Possible past/present infection |
| + | – | Prevalence of – Neutralizing antibodies |
Possible positivity due to vaccination* |
| – | + | Prevalence of – Non-neutralizing antibodies |
Possible past/present infection** |
| – | – | Absence of antibodies (or not detectable) | Possible absence of contact with the virus |
* If absence of Anti-NC antibodies is confirmed
** If presence of Anti-NC antibodies is confirmed after retesting
COVID-19 Anti-S1 Total Ab
Immunoturbidimetric test for the semi-quantitative determination of human total antibodies (IgA, IgG and IgM) specific for Spike protein S1 of SARS-CoV-2 in human serum
Intended Use
The COVID-19 Anti-S1 Total Ab assay is an in vitro diagnostic test for the qualitative and semi-quantitative determination of total antibodies (IgA, IgG and IgM) to SARS-CoV-2 in human serum. The test is intended for use as an aid in identifying individuals with an adaptive immunoresponse to SARS-CoV-2, indicating recent or prior infection.
COVID-19 Anti-NC Total Ab
Immunoturbidimetric test for the semi-quantitative determination of human total antibodies (IgA, IgG and IgM) specific for Nucleocapsid protein of SARS-CoV-2 in human serum
Intended Use
The COVID-19 Anti-NC Total Ab assay is an in vitro diagnostic test for the qualitative and semi-quantitative determination of total antibodies (IgA, IgG and IgM) to SARS-CoV-2 in human serum. The test is intended for use as an aid in identifying individuals with an adaptive immunoresponse to SARS-CoV-2, indicating recent or prior infection.
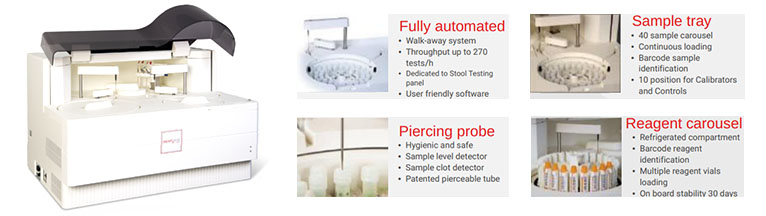
Analyzer
- Walk-away system
- Immunoturbidimetric method
- Throughput up to 270 tests/h
- First result after 10 min only
- Full traceability (barcode)
- User friendly and complete software
- External Laser printer
Kit Contents
| COVID-19 Anti-S1 Total Ab | COVID-19 Anti-NC Total Ab | |
|---|---|---|
| Method | Particle enhanced turbidimetric immunoassay | Particle enhanced turbidimetric immunoassay |
| Measurement | Semi-quantitative | Semi-quantitative |
| Target | Antibodies to Spike RBD S1 (S1) viral protein of SARS-CoV-2 | Antibodies to Nucleocapsid (NC) viral protein of SARS-CoV-2 |
| Ab isotype | Total (IgA, IgG, IgM) | Total (IgA, IgG, IgM) |
| Source of the antigen | Recombinant antigen protein | Recombinant antigen protein |
| Ab Classification | Prevalence of Neutralizing antibodies | Prevalence of Non-neutralizing antibodies |
| Origin of the Ab | Past/present infection Vaccination* |
Past/present infection** |
| Sample | Serum | Serum |
| Reference values | Negative Value: <0.80 AU/mL | Negative Value: <2.20 AU/mL |
Benefits of the kits
- The quick solution for evaluating immunity status of patients
- High throughput testing on fully automated systems
- Applicable on most common clinical chemistry analyzers
- Ideal solution for screening of community and population
- Useful to verify immune response after vaccination
- Better precision and repeatability than rapid card tests
- High performance, ready-to-use reagents with minimized interferences, long shelf life and onboard stability
- Perfectly matched liquid-stable reagents, calibrator and controls
- Very good correlation with PCR results
- Avoid interference due to Biotin and HAMA
- Environmental sustainable product from ISO 14001:2015 certified company
- > 35 years experience in immunoturbidimetry assay

